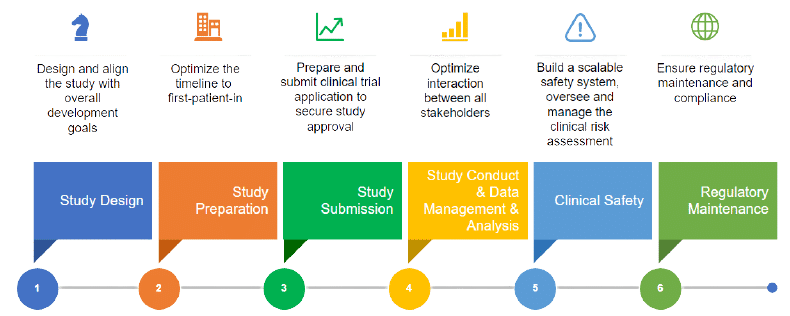
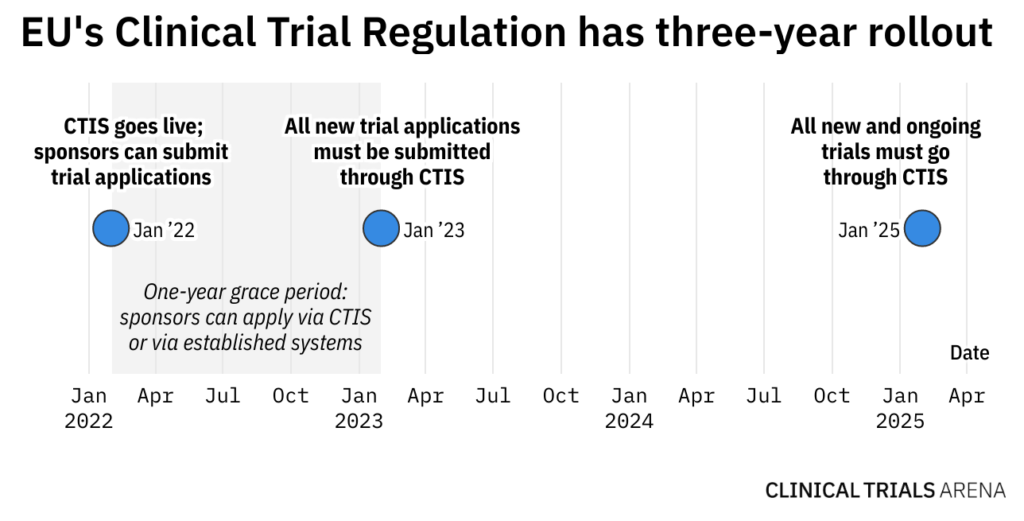
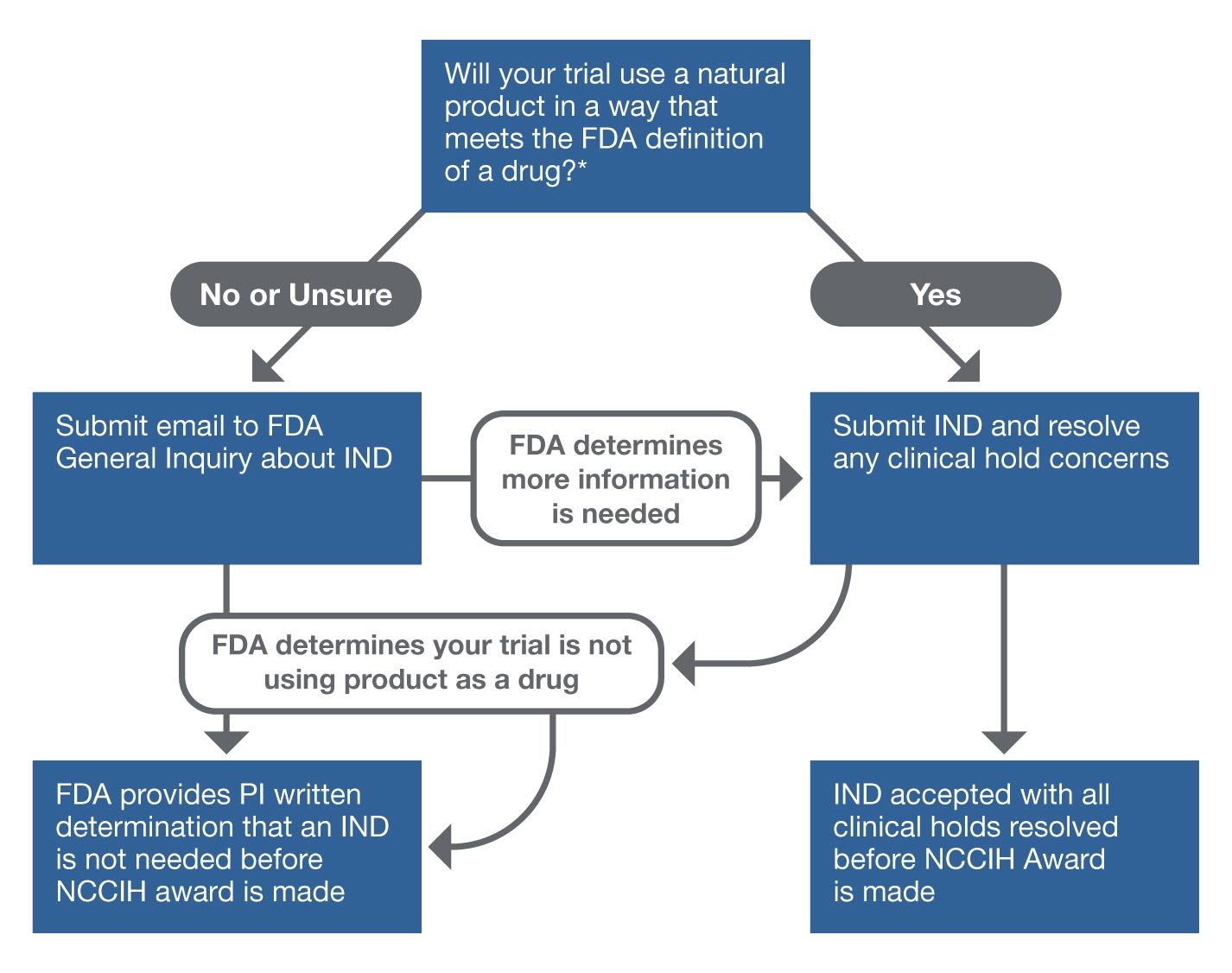
Radiation Therapy Digital Data Submission Process for National Clinical Trials Network - International Journal of Radiation Oncology, Biology, Physics

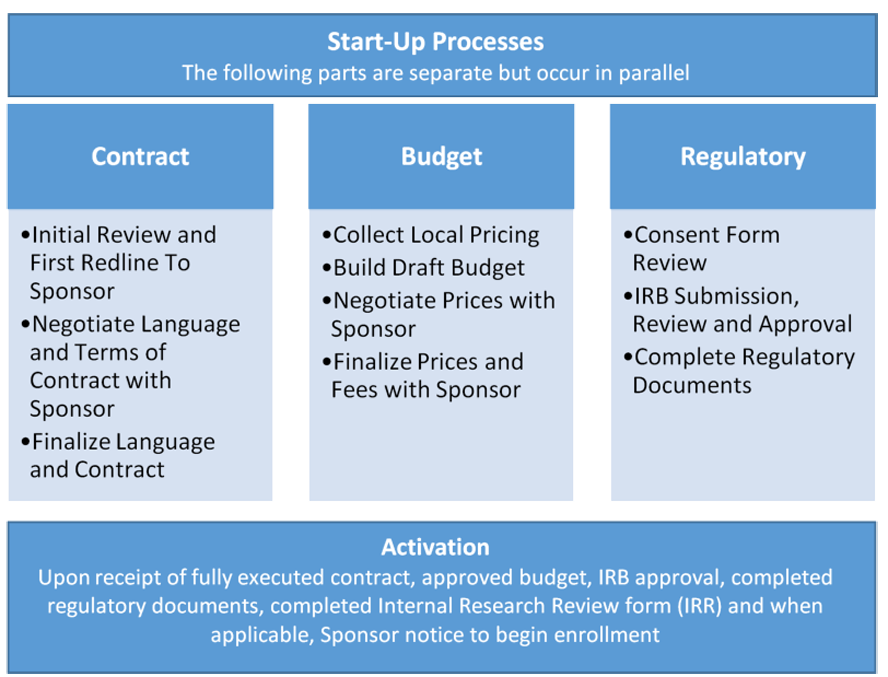
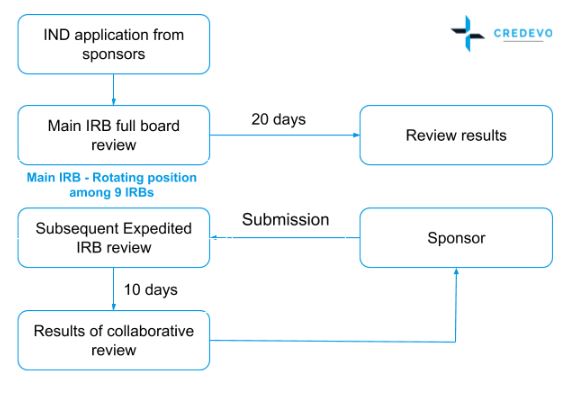
Regulatory submission steps for our clinical research group in Brazil. | Download Scientific Diagram