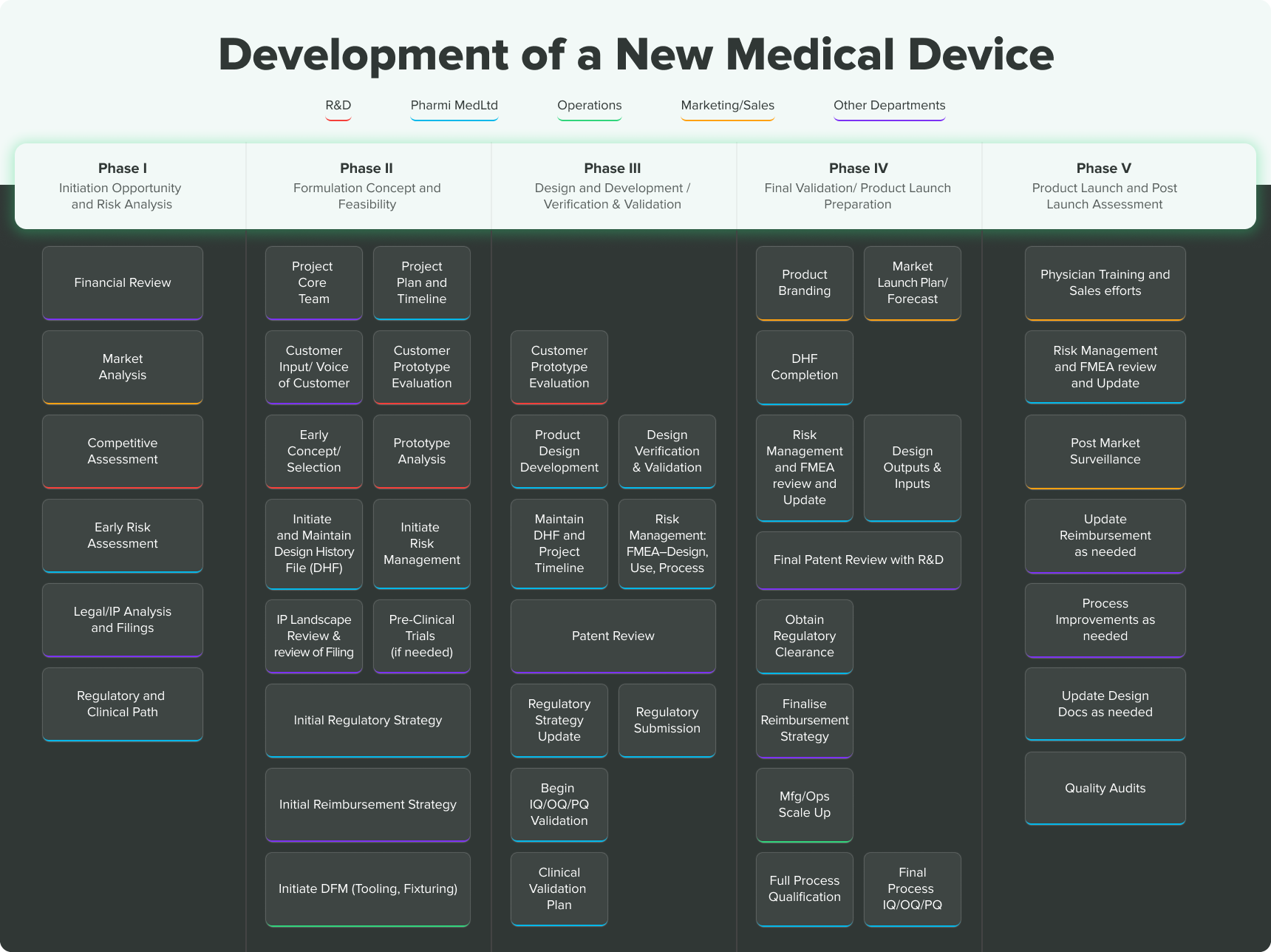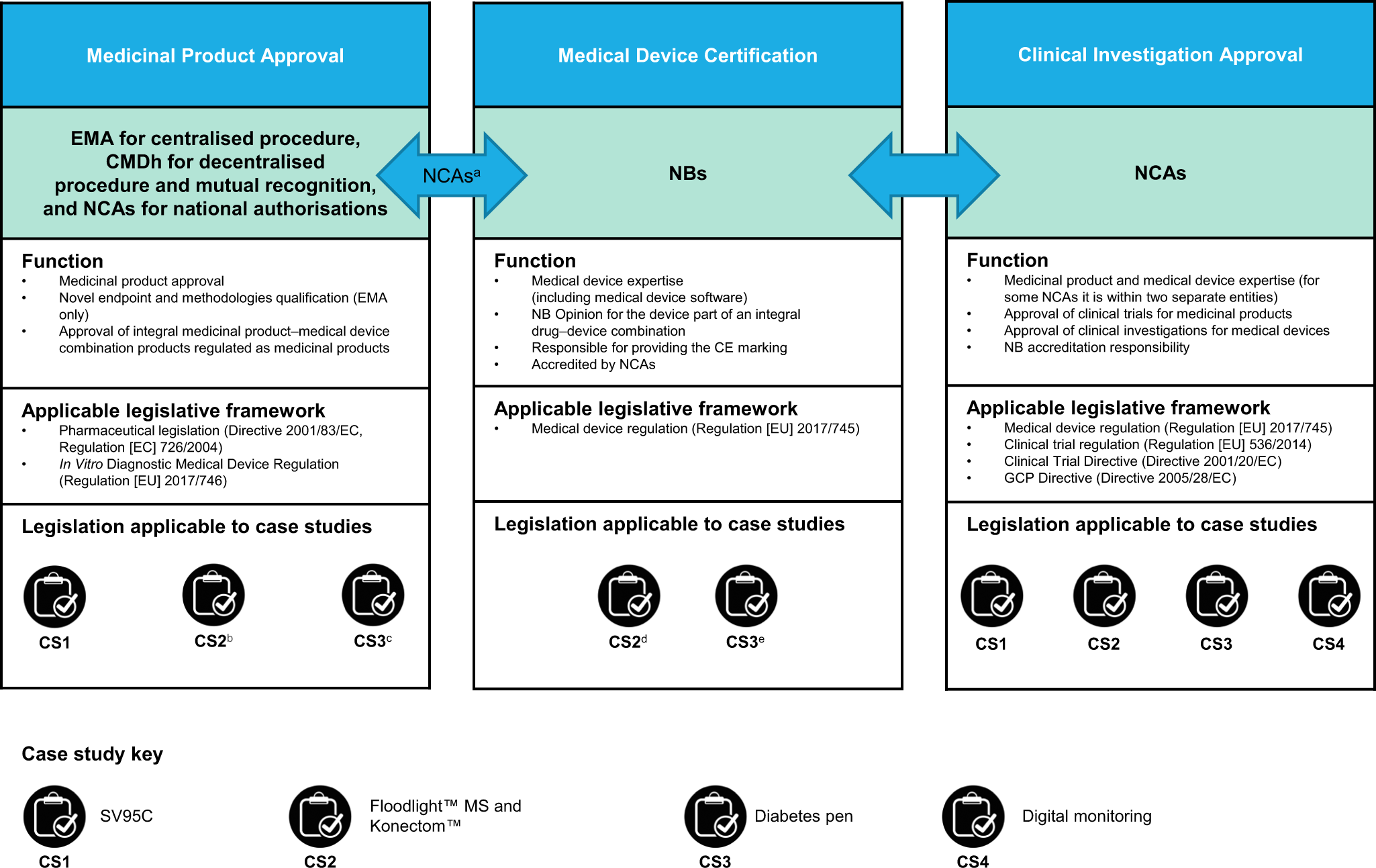
Evolving regulatory perspectives on digital health technologies for medicinal product development | npj Digital Medicine
Software as a Medical Device (SAMD): Clinical Evaluation - Guidance for Industry and Food and Drug Administration Staff

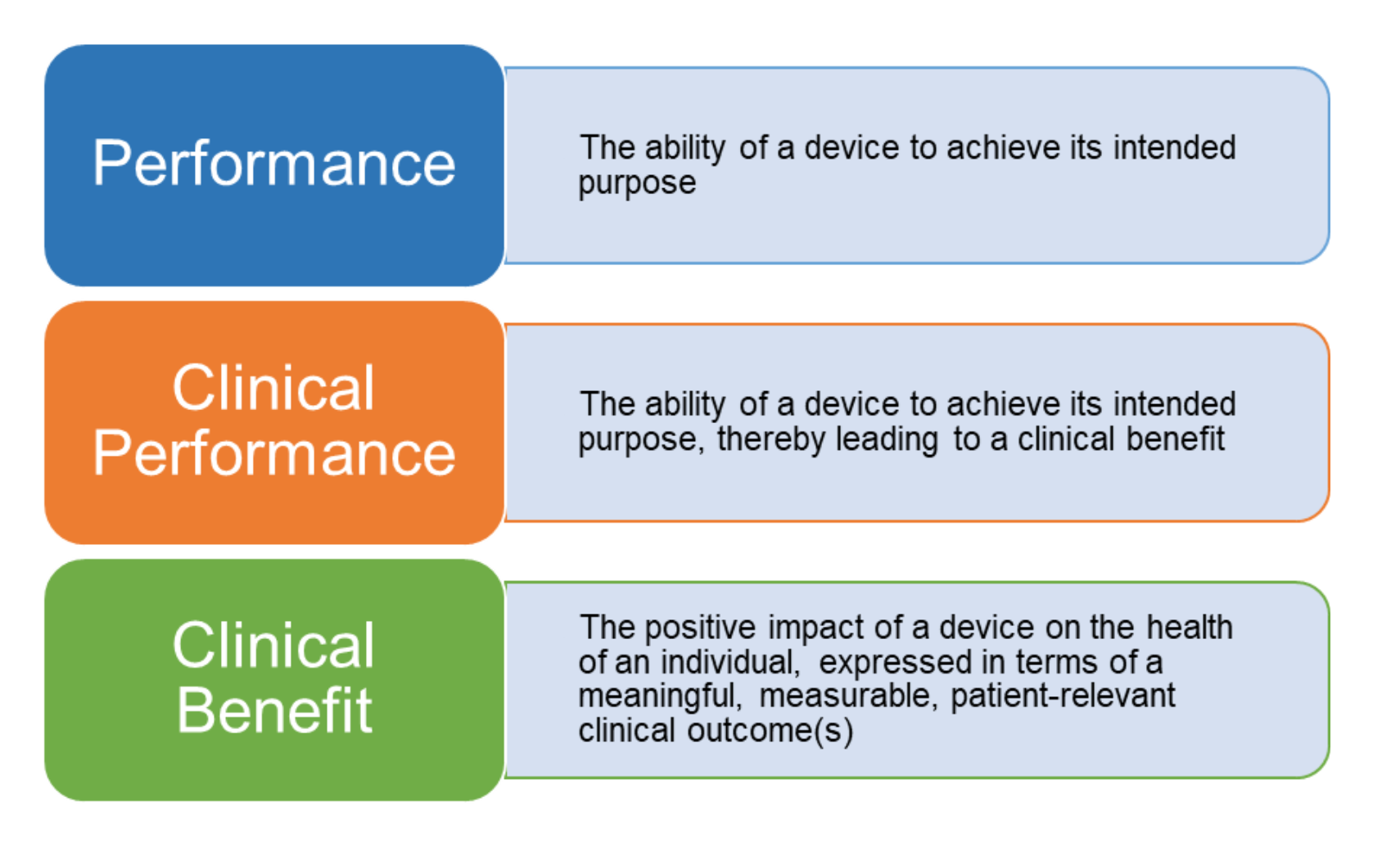
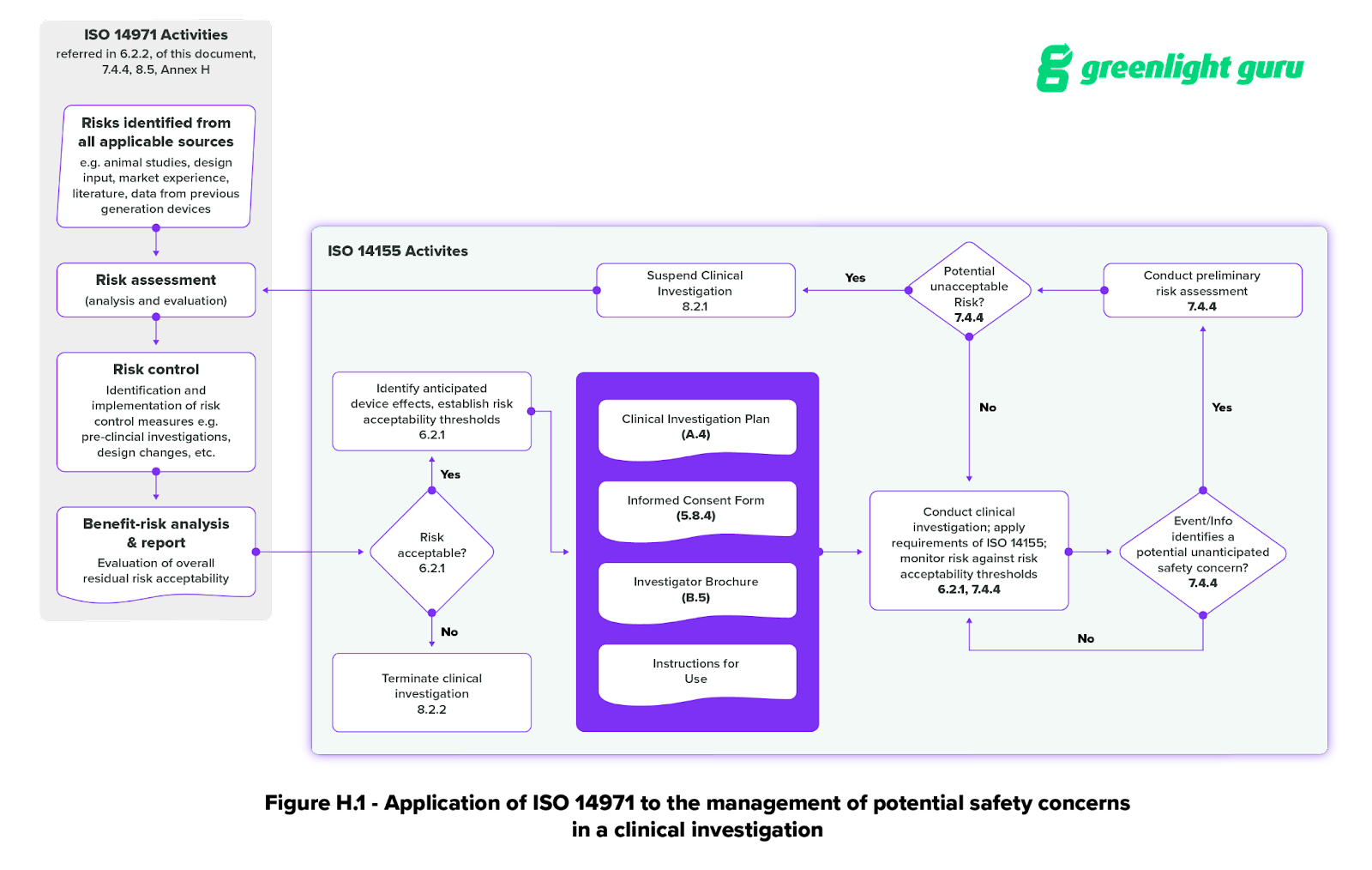
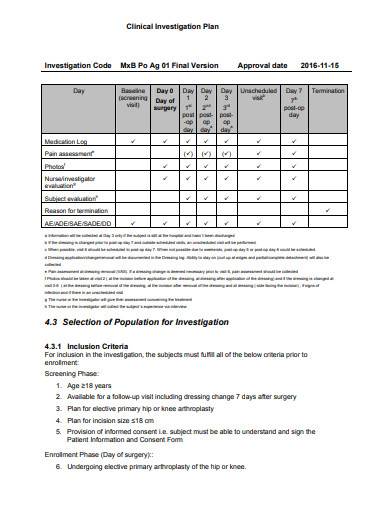
ANSI/AAMI/ISO 14155-2:2003 - Clinical investigation of medical devices for human subjects - Part 2: Clinical investigation plans
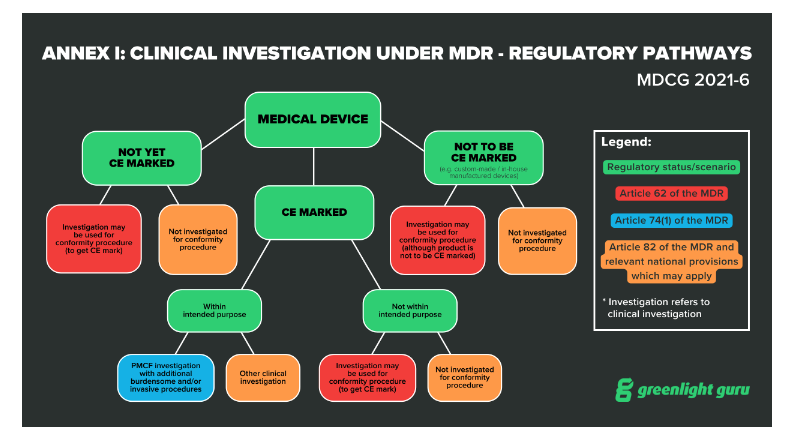
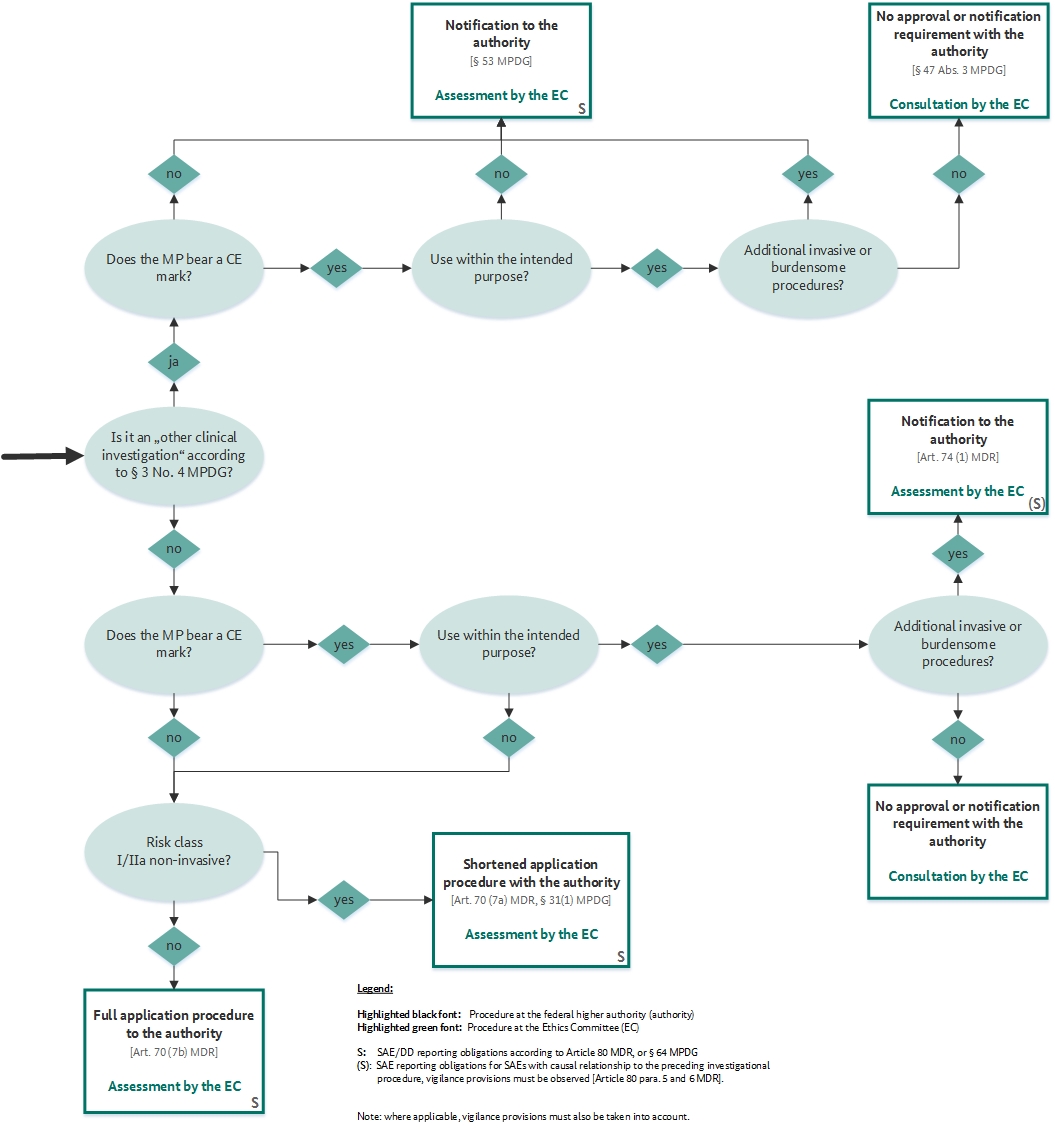
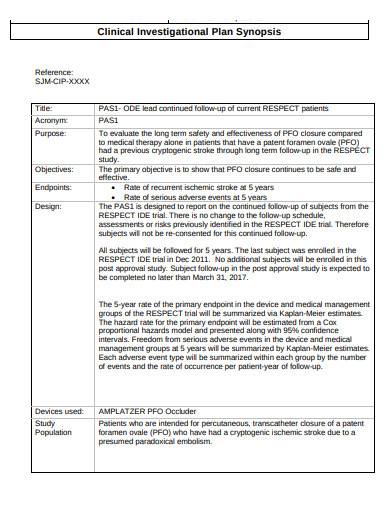
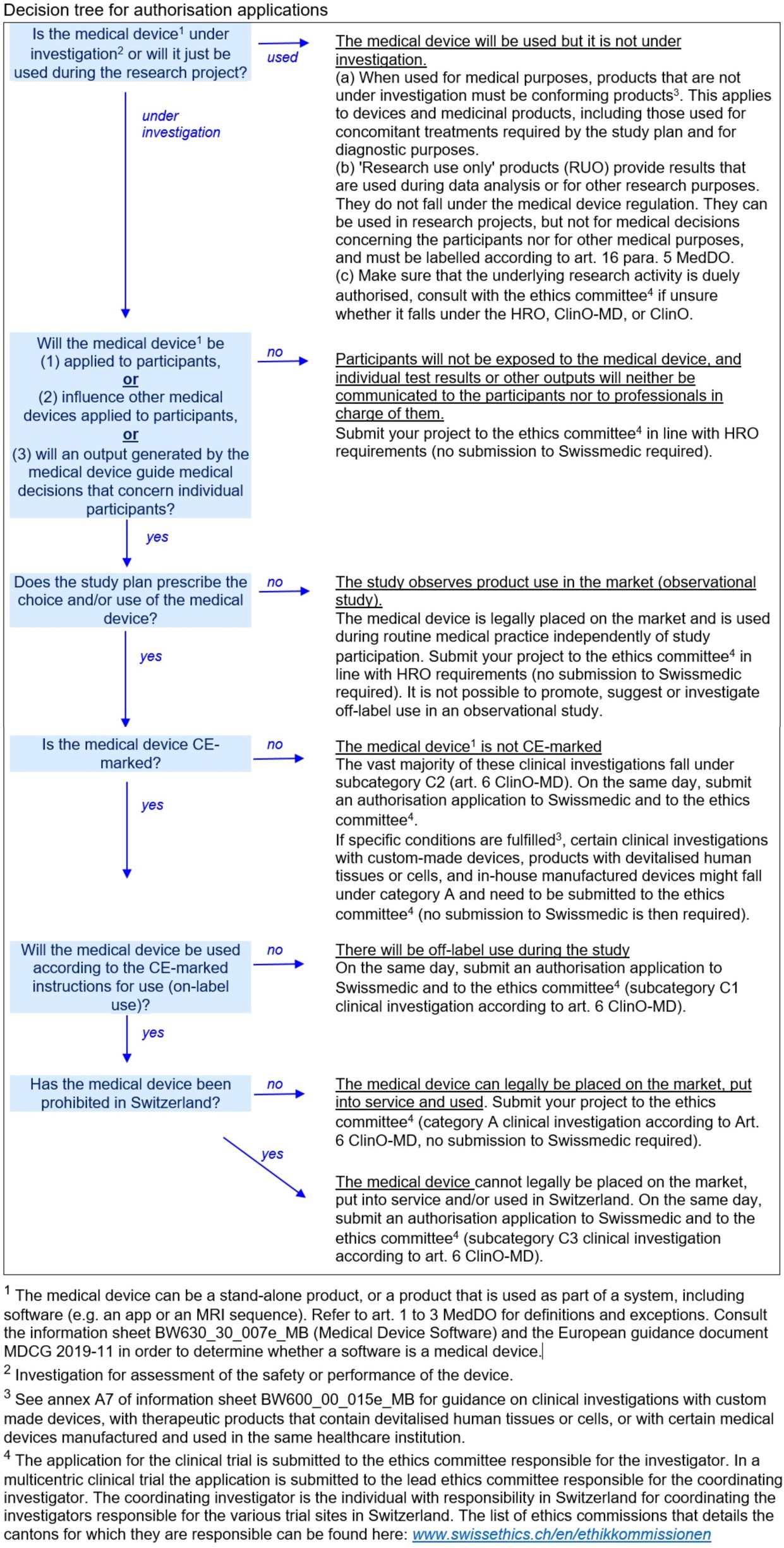
GUIDELINES ON MEDICAL DEVICES GUIDELINES FOR COMPETENT AUTHORITIES FOR MAKING A VALIDATION/ASSESSMENT OF A CLINICAL INVESTIGATIO

ONORM EN ISO 14155-2:2009 - Clinical investigation of medical devices for human subjects - Part 2: Clinical investigation plans (ISO 14155-2:2003)